All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional.
The GvHD Hub uses cookies on this website. They help us give you the best online experience. By continuing to use our website without changing your cookie settings, you agree to our use of cookies in accordance with our updated Cookie Policy
Introducing

Now you can personalise
your GvHD Hub experience!
Bookmark content to read later
Select your specific areas of interest
View content recommended for you
Find out moreThe GvHD Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the GvHD Hub cannot guarantee the accuracy of translated content. The GvHD Hub and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
ECP in adult patients with GvHD: A real-world experience
Bookmark this article
Extracorporeal photopheresis (ECP) can be used to treat adult patients with steroid-refractory or steroid-dependent graft-versus-host disease (GvHD).1 The optimal schedule for ECP treatment is not defined clearly, and it is thought that using an off-line treatment schedule, rather than the traditional in-line regime, may allow for fewer sessions as well as a reduced amount of disposal apheresis instrumentation.1
Here, we summarize key findings regarding real-world experience in ECP for adults with GvHD, published by Canto et al.1 in Transplantation and Cellular Therapy.
Study design1
- Patients were eligible for inclusion if they had been diagnosed with acute GvHD (aGvHD) or chronic GvHD (cGvHD) and had previously undergone at least one off-line ECP session.
- ECP was indicated for these patients after the failure of steroid therapy.
- In total, 82 adult patients ≥18 years were included for analysis, and 1,382 ECP procedures were performed.
- Off-line ECP procedure:
- 28 patients with aGvHD received: 2 procedures per week for 2 weeks, followed by 1 procedure per week for 2 weeks, and then 1 procedure biweekly for 5 months;
- 54 patients with cGvHD received: 1 procedure per week for 1 month, followed by 1 procedure biweekly for 5 months;
- ECP was tapered for each patient based on the individual’s GvHD response and introduction or withdrawal of immunosuppressants.
- Steroids were tapered by 0.2 mg/kg prednisone-equivalent per day, every day for 5 days in aGvHD, and 20–30% every 2 weeks in cGvHD.
- Cumulative incidences of response, including complete response (CR) and partial response (PR), were compared among patients grouped by different baseline, apheresis, and disease characteristics.
Key findings1
In the aGvHD cohort, patients who responded to ECP treatment had a 1-year overall survival (OS) of 67.5%, whereas patients who did not respond had an OS of 26.0% (p = 0.037). Patients in the cGvHD cohort who responded to ECP treatment had a 1-year OS of 85.0%, and those who did not respond had an OS of 85.7% (p = 0.57). Table 1 summarizes the response rates and median duration of response of patients who received ECP treatment.
Table 1. Efficacy outcomes following ECP treatment*
|
|
aGvHD cohort |
cGvHD cohort |
|---|---|---|
|
Patients who achieve CR |
16 (57%) |
21 (39%) |
|
Patients who achieve PR |
1 (4%) |
26 (48%) |
|
Median duration of treatment |
3 months |
7 months |
|
Median duration of response |
4.1 months |
14.3 months |
|
Median number of ECP procedures |
11.5 |
17 |
|
aGvHD, acute graft-versus-host disease; cGvHD, chronic graft-versus-host disease; CR, complete response; ECP, extracorporeal photopheresis; PR, partial response. |
||
Below, Figure 1 illustrates complete response rates in patients with aGvHD and cGvHD, focusing on skin, gut, and liver involvement.
Figure 1. Patients who achieved CR, in specific organs, after ECP treatment*
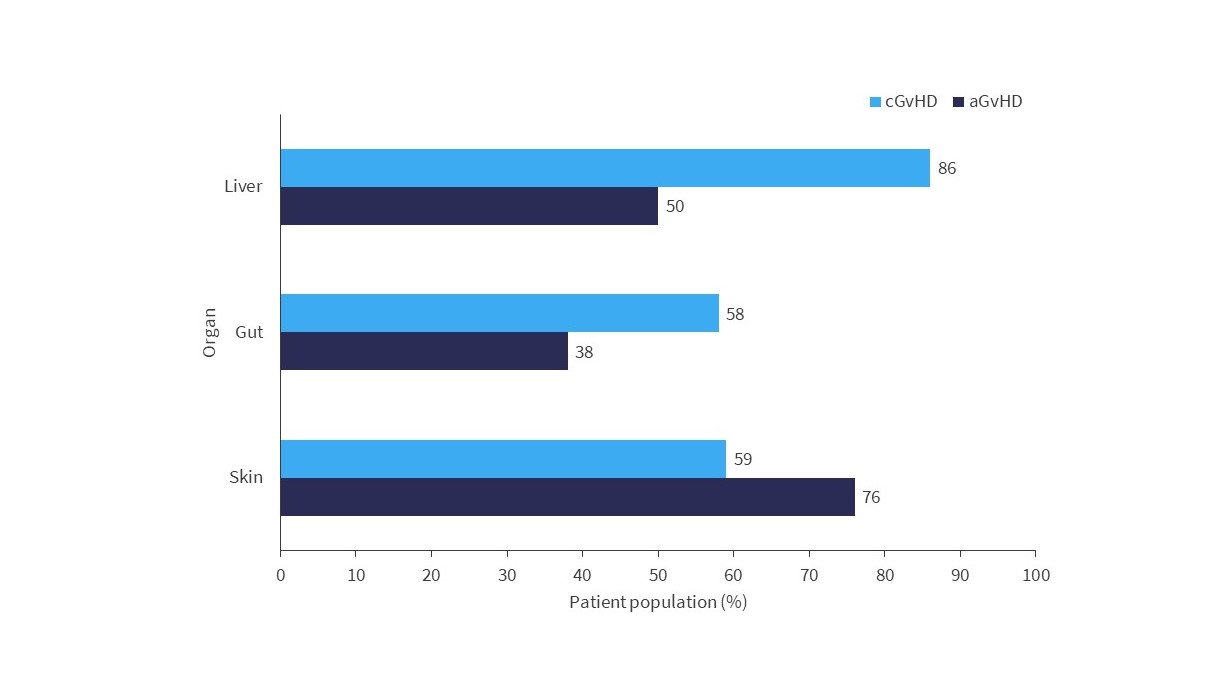
aGvHD, acute graft-versus-host disease; cGvHD, chronic graft-versus-host disease; CR, complete response; ECP, extracorporeal photopheresis.
*Adapted from Canto, et al.1
Incidences that prevented ECP procedures included issues with venous access, autologous mononuclear cell collection, apheresis instrumentation errors, and psychological intolerances (1.4%); the remainder of the ECP procedures (98.6%) were completed successfully.
|
Key learnings1 |
|
- Canto PA, Caballer JS, Pell-Ilderton CS, et al. Real-world experience in extracorporeal photopheresis for adults with graft-versus-host disease. Transplant Cell Ther. 2023;29(12):765e.1-756e.8. DOI: 1016/j.jtct.2023.09.001.
Related articles
Newsletter
Subscribe to get the best content related to GvHD delivered to your inbox






